The Most Common Nanodropper FAQs From Eyecare Professionals, Answered Here
Updated: June 5, 2023
Calling all optometrists and ophthalmologists! Are you considering bringing the Nanodropper to your clinic? Interested in using it on your in-clinic drops or offering it to patients? We’ve answered the most common questions asked by eyecare professionals. Read on to learn more about safety, efficacy, partner clinic info, and much more!

1. Which bottles can I use it on in my clinic?
The Nanodropper is compatible with almost all in-clinic drops (proparacaine, Paremyd, tropicamide, phenylephrine, etc) except Fluress / Fluorescein.
Compatibility with the Nanodropper Adaptor depends on the diameter of the threads on the bottle neck (9/16 in, ~15mm). Any other bottles in your clinic that have the same size thread diameter as the bottes listed above will be compatible as well!
You can also visit our compatibility page for more information.
2. Is the Nanodropper reusable? If you can’t resterilize it, is it cost effective?
The Nanodropper Adaptor is a Class I, FDA-listed, sterile medical device. The Nanodropper should not be transferred between bottles of eyedrops. We recommend using one adaptor per medication to prevent risk of contamination and injury. Since the Nanodropper triples the life of your eyedrop bottles, the savings will pay for the adaptor and much more. If your bottle costs $10 or more, the Nanodropper will pay for itself.
Please do not attempt to try to clean the Nanodropper Adaptor. We use gamma radiation for sterilization to promote a high level of patient safety. The Nanodropper cannot be autoclaved.
3. What does it mean to be a Partner Clinic?
Our network of Partner Clinics is growing and your clinic can be a part of it!
Benefits include:
- Wholesale pricing when ordering in quantities of 20
- Prices starting at 37% off $19.99 MSRP ($12.50/unit)
- Use Nanodropper for in-clinic drops
- Triple your in-clinic drops and save money!
- Insurance against drug shortages and price spikes
- A clinic of 10 providers will save approximately $15,000 per year on reduced drug waste
- Free advertising on our website
- Your clinic will be listed and mapped for patients in your area to buy directly from your clinic
- Complimentary clinic display including supplementary patient materials!
- Let patients see, touch and demo the Nanodropper before purchasing!
- Includes a patient benefits cheat sheet for staff
- Complimentary patient brochures upon request
4. How can my clinic become a Partner Clinic?
It’s a really simple process! If you want to use the Nanodropper in your clinic and offer it in your clinic to your patients, please follow the instructions to fill out the Clinic Account Creation form here. The form takes just about 2 minutes to complete!
5. Does the medication expire since the bottles last much longer with the Nanodropper?
In general, preservative-containing medications are safe to use up to the printed expiration date on the bottle, regardless of when the bottle is opened. In fact, a recent statement by EyeSustain, supported by multiple ophthalmic organizations, encourages providers to use multi-use eyedrop bottles until their printed expiration date to reduce medication waste.
The manufacturer’s storage and disposal recommendations should always be followed, so we encourage you to check this information for each drug you plan to use or recommend the Nanodropper for.
6. What is the adaptor made of? Can it cause allergic reactions to certain people?
The Nanodropper is made out of high density polymers (grey base and cap) and medical-grade silicone (blue tip). Both materials are entirely inert and do not cause allergic reactions.
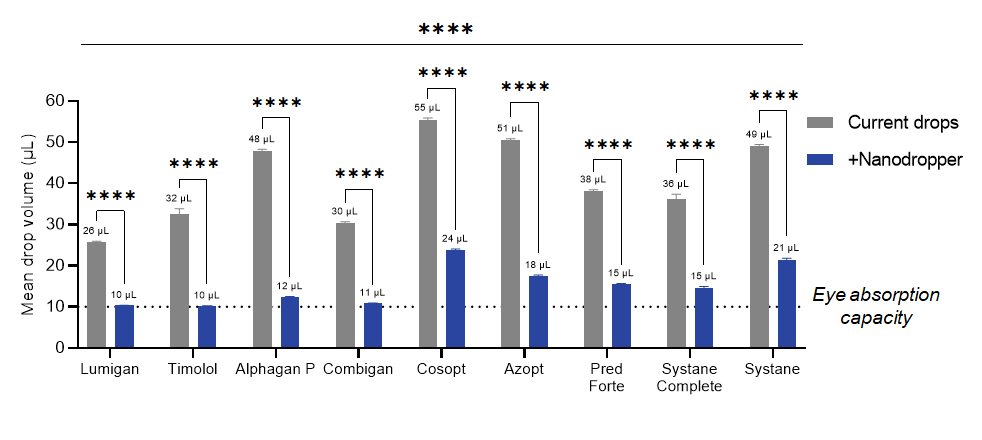
7. How much smaller does it make the drops?
Drops with the Nanodropper are about 3 times smaller than the original drops. Please see our benchtop data collected in collaboration with the University of Colorado Anschutz Medical Campus, Department of Ophthalmology for additional drug-specific drop size information.
8. Are small drops just as efficacious?
We have a number of studies on the efficacy of smaller drops listed in our whitepaper. The efficacy of smaller drops as well as the eye absorption capacity (7-10μL) has been extremely well studied. Many of the major pharmaceutical companies are also on record stating that drop size is not a medical dosing issue.
Please see more about our regulatory and quality information here. We have a number of clinical trials completed and in progress that you can review at this link. Contact us if you are interested in running a trial with the Nanodropper.
Have a question not listed here? Email it to pro@nanodropper.com or call 507-405-5676. At Nanodropper we are committed to decreasing barriers to patient care, and that care starts with eyecare providers. Help us increase patient access by becoming a Partner Clinic with Nanodropper!